Table Of Content
Join 200,000+ other medical device professionals outperforming their peers. Conduct tests according to the plans you made in the preparation phase. Design verification tests whether a product can achieve its purpose and identifies any glitches. Test Case – A documented procedure, used to test that a system meets a requirement or collection of requirements. Quality Assurance – Team members tasked with ensuring product quality or process integrity. Take time to define how you want tools to support your team before you choose.

Use design testing to prioritize brilliantly
The FDA has established specific requirements for design verification and validation to ensure the safety and effectiveness of medical devices. These requirements are outlined in various regulatory documents, including the FDA’s Quality System Regulation (QSR) and the Design Control Guidance for Medical Device Manufacturers. Although design verification and design validation have very different meanings, it’s easy for professionals to incorrectly interchange the use of the terms. This process helps product development teams spot design flaws and rectify them, reducing risks such as product failure, therefore saving resources and time.
Healthcare Branding

On the other hand, you should also verify that designs meet the needs of their users. This means that the product is implemented to spec and is measured for quality in terms of usability and accessibility. Design verification can be done through usability testing and quality assurance to ensure that product experiences align with user needs and expectations. Include test engineers early in development planning to make sure requirements and design are clear, complete and testable.
How Our LA Graphic Designers Deliver Unique Services
When faced with a complex problem space or opportunity area, countless unknown factors come into play. Designers can base decisions on assumptions, but that runs the risk of ignoring actual user needs. But post-market information, such as nonconformances, customer feedback, or corrective and preventive actions (CAPA) might necessitate a change to the design after your product is on the market. For instance, one of your user needs may be that the user must be capable of operating the device with one hand. But that single user need may generate several design inputs related to size, weight, and ergonomics, just to name a few.
Finally, the team combines the look-like and work-like prototypes into one form factor, selects the main function features, and identifies any design issues that need fixing. Make sure the information you gather trickles through to everyday design choices and each stage of the design process. Card sorting tests the design, usability, and information architecture of your site or product page. You’ll ask participants to move cards into the themes or topics they think is the right fit and you may ask them to come up with labels. Potential risks like single-source supplies—when a component is restricted to be made by a single selected CM—will be identified through risk management protocols such as FMECA, QA/QC, and FAI.
Siemens launches three-part Veloce CS system for chip validation - FierceElectronics
Siemens launches three-part Veloce CS system for chip validation.
Posted: Tue, 20 Feb 2024 08:00:00 GMT [source]
A typical design process for a complex electromechanical product includes multiple concepts, each one supported by a stack of exploration sketches, a series of physical mockups, and a set of 3D renderings. In this guide, you’ll learn how rapid prototyping fits into the product development process, its applications, and what rapid prototyping tools are available to today’s product development teams. Finally, don’t forget that design validation must include packaging and labeling, too.
Creating effective design verification and validation plans
Says Megan, “Early development of test methods can shed light on technology issues before they become major obstacles.” Early test development can also provide test tools. These can then be used to speed the product development process as well as provide test evidence during formal testing. Design verification provides evidence (test results) that the design outputs (actual product) meet the design inputs (product requirements and design specifications). Depending on the item being verified, a test case or test suite would be run, or an inspection or analysis done to provide the required evidence.
It’s a potent method but takes time for the data to aggregate and needs developers to help you implement the A/B testing. Heatmaps are visual representations of how users interact with your product. That’s why once in doubt, have your designer friend or a colleague take a look at your work. See how design choices, interactions, and issues affect your users — get a demo of LogRocket today.
‘Customer-Centric Design, Validation, Production Processes To Raise End-user Experience’ - Mobility Outlook
‘Customer-Centric Design, Validation, Production Processes To Raise End-user Experience’.
Posted: Mon, 05 Feb 2024 08:00:00 GMT [source]
A design validation plan is a structured framework used to guide the process of validating a product’s design to ensure it meets the intended specifications and requirements. It’s the roadmap for systematic testing and confirming that the design is reliable. Code reviews involve a meticulous examination of the software code to identify any potential errors, inefficiencies, or inconsistencies. This process ensures that the code adheres to established coding standards and best practices. Unit tests, on the other hand, focus on testing individual units or modules of the software to verify their functionality and performance in isolation. This helps identify any flaws or defects within the individual units before they are integrated into the larger system.
Experience the #1 QMS software for medical device companies first-hand. The verification process applies to every feature or specification of the product. V&V Plan – A plan defining the requirements to be verified and validated, and the manpower, responsible individuals, tools, methods, resources, and schedule for the V&V effort.
There might be tolerance issues with a mating part under certain conditions. Or a lead user group could come up with a new high-priority requirement forcing the designers into another round of development. Etienne Nichols is a Medical Device Guru and Mechanical Engineer who loves learning and teaching how systems work together.
You must take enough time to verify and validate a product to ensure you solve all issues. Everyone has time limits, but verification and validation should be priorities. You need to adapt the design verification and validation process to the needs of different industries.
Includes final report, with test results and traceability, ready for regulatory review. By the way, the table above also shows the traceability between user needs and test cases. This trace matrix provides part of the V&V evidence that the FDA requires. Interviews can be a stressful experience, but effective communication skills can help you ace the interview and land the job. Furthermore, your design should incorporate fewer changes to allow estimating each design decision separately. Otherwise, it will be difficult to establish what’s working and what’s not.
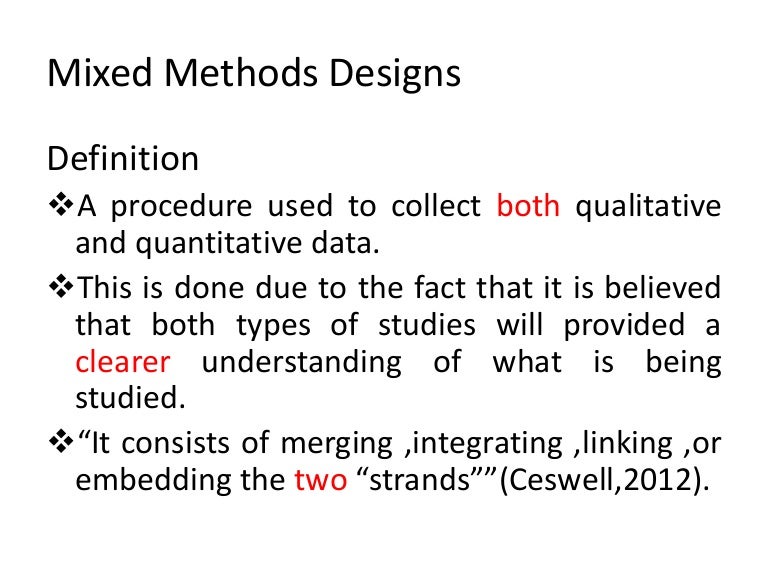
No comments:
Post a Comment